Влияние экстракта грибов шиитаке (Basidiomycetes) на половое поведение млекопитающих: Microtus socialis
Зоренко Т. А., Мотмиллере И. З., Матюшкова Н. А. Влияние экстракта грибов шиитаке (Basidiomycetes) на половое поведение млекопитающих: Microtus socialis. Лабораторные животные для научных исследований. 2018; 3. https://doi.org/10.29296/2618723X-2018-03-03
Резюме
В последние годы в разнообразных биологических исследованиях часто используют полевок (отряд Rodentia), которые в силу своих поведенческих и физиологических характеристик представляют удобный лабораторный объект. Результаты проведенных экспериментов свидетельствуют о высокой афродизиатической активности грибов шиитаке. Трехнедельный рацион с добавлением водного экстракта шиитаке привел не только к восстановлению половой мотивации у самцов с изначально пониженным либидо, но и к повышению числа эякуляций в 1,5 раза, а к общей продолжительности спаривания – в 1,8 раза (на 19,4 мин). Контрольный рацион с добавкой отрубей (Контроль-1) или экстракта грибов вешенок (Контроль-2), хотя и восстановил влечение к самке и осуществление спаривания, но не изменил показателей спаривания, более того, в Контроле-2 (вешенка) они были ниже, чем в усредненной видовой норме. У самцов с проявлением изначально нормальной половой мотивации афродизиатический эффект водного экстракта шиитаке был еще значительнее. Не только статистически достоверно возросли такие показатели спаривания, как число эякуляций и общая продолжительность спаривания, но и количество толчков, которые определяют повышенную генитальную стимуляцию, что важно для более успешного оплодотворения самки и получения большего числа потомков. Экстракт вешенки также вызвал увеличение числа эякуляций, хотя и значительно меньшее, чем у самцов, получивших экстракт шиитаке. Кроме того, он не повлиял на другие показатели спаривания. Механизм действия грибов на половую активность животных до сих пор не установлен. Обсуждаются различные гипотезы этого влияния. Возможно, не один, а многие компоненты грибов (лентинан, Zn, карнитин и др.) создают кумулятивный эффект, вызывая улучшение активности половых желез самцов, а также качества их спермы (плотность и подвижность сперматозоидов), в результате чего повышается половая активность, т.е. либидо.
Introduction
There are more than 50 species of mushrooms with a wide range of therapeutic properties, which are referred to as medicinal mushrooms, including the most popular Ganoderma lucidum (Curt.: Fr.) P. Karst. (reishi), Cordyceps spp. (caterpillar fungus), Lentinula edodes (Berk.) Pegler (shiitake) and Pleurotus ostreatus (Jacq. ex. fr) Kummer (oyster mushroom). Shiitake (Lentinula edodes) is an edible mushroom cultivated worldwide with medicinal properties [1, 2]. Since the second half of the last century, their popularity has been growing worldwide with the development of cultivation techniques and with increasing awareness of their therapeutic properties supported by scientific research. Lentinula edodes has a number of therapeutic properties – strengthens the immune system, exhibits antitumor activity, prevents cardiovascular disease and diabetes. It also possesses antioxidant, anti-inflammatory, liver protective properties, and helps with bronchitis and allergies. Orient medicine yet for a long ago uses the preparations made of shiitake mushrooms for treatment of an impotence and increase of sexual activity and libido of the person. However, controlled scientific studies on the potential of medicinal mushrooms in the treatment of certain animal disease are scarce. It displayed activity against viral, bacterial, fungal and parasitic infections, such as candidies, common cold and influenza [3, 4].
Last year’s data about the impact of shiitake mushrooms on animals’ individual development and their reproduction were accumulated. As models laboratory rats, rabbits and voles were used [5–9] as well as hens [10] and rainbow trout (Oncorhynchus mykiss) [11]. In comparison with other effects, there is little information of the influence of shiitake on animal reproduction [9, 12–14].
Experimental evidence of the aphrodisiac effect of shiitake on mating is practically absent. Recently, as a model species the fruit fly Drosophila melanogaster was used to prove the influence of shiitake on copulatory activity [13]. However, despite the many experimental merits of this species, its stereotype of mating is very different from that of mammals. It should be noted, that copulation stereotypes have many common patterns only in rodents and primates [15]. Therefore, experimental data on the influence of the feeding with shiitake upon the sexual activity and libido are especially important. This study was made to explore the aphrodisiac effect of the feeding with shiitake on copulatory behaviour
Materials and methods
The study was conducted from August to November 2004.
Preparation of aqueous extracts of mushrooms
Extracts of mushrooms were prepared in the laboratory of genetics of plants and microorganisms of the University of Latvia. Fresh fruiting bodies (200 g) of L. edodes strain DSM3565 (German Collection of Microorganisms and Cell Cultures) were homogenised and extracted with distilled boiling water (1 L) for 15 h at 80°C. The suspension was centrifuged to remove the insoluble matter at room temperature [16]. Approximate crude polysaccharide content in shiitake fruiting body extract was 3.2 gram per litre. The oyster mushrooms extract supplement was prepared the same way. 10 ml of each extract was added to 5 g of wheat bran: accordingly, shiitake extract supplement (SES), water (Control-1) and oyster mushrooms extract supplement (Control-2). The animals received the bran with extracts in Petri dishes, and they ate directly from them, so the feeding was controlled visually. Animals received a food supplement for three weeks.
Rearing of voles
Progenitors of social vole Microtus socialis (Rodentia) colony at the University of Latvia were captured in Kalmyck region (Russian Federation). This colony had nine years breeding history in vivarium. The social vole was chosen as a test animal since its reproduction has been extensively studied (i) [14, 17]. It has relatively recent history of breeding in laboratory and may exhibit ampler reactivity against bioactive substances (ii). Adult animals (average body’ length 96 mm) are significantly smaller than rats, guinea pigs or rabbits, what permits maintenance of larger animal colony in condensed laboratory space (iii).
The animals were kept in standard laboratory cages in parent groups until a month and a half, the males housed in separate cages. Wood sawdust and hay were used as litter material. Vegetables and grains mixture were the basic nutrition of the voles. Temperature in the vivarium was 20±2oC and light/dark cycle of 12 L/12 D was maintained. The keeping of animals satisfied the requirements of Federation of European Laboratory Animal Science Associations (FELASA, certificate С–category).
Experimental Design
Our study consisted of two parts. Through the first part during 10 days, 47 males aged 3 months were tested for mating ability. Of these, 17 males (36.2%) showed no interest in females, and despite their receptivity, mating did not begin. The non-copulated animals were randomly assigned into three specified experimental groups: in the first group, six males received a food SES supplement, in the first control group, six males were given bran with water and in the second control group, and five males received an oyster mushrooms extract supplement. After 7 days of feeding, the males were tested for sexual capacity. A second test with the same males was conducted two weeks later, during which they received a food supplement (total three weeks).
Through the second part of the study – 30 males were randomly divided into three experimental groups, 10 males per group. A second test for sexual capacity was repeated after 3 weeks of adding supplements (SES, Сontrol-1 and Сontrol-2) into the diet.
A mating ability test
Testing of males’ behaviour was carried out in experimental cage (its dimensions 600×450×300 mm). The duration of the experiment was determined by the criteria of sexual satisfaction. The experiment ended when the criteria for satiety in males, indicated by 30 min without copulation, was achieved. If the males in one of the series of mating (usually the last) did intromissions, but did not finish mating with ejaculation, or did not start mating, the experiment lasted 60 minutes. During the experiments, direct observations and filming were conducted.
Only sexually experienced females aged 3–4 months were used in the experiments. The phase of the sexual cycle was controlled with the help of the cytology of the vaginal smears [18].
Copulation of voles consists of a series of mounts, alternating with period of rest. Each series includes mounts with vaginal insertion (intromissions) and intravaginal thrusts and mounts with insertion, thrusting, and sperm transfer (ejaculation). The following indices were recorded: Latency (L) – period from introduction of the female until the first intromission; Ejaculation Frequency (EF) – number of ejaculations preceding attainment of the criteria for satiety in males, indicated by 30 min without copulation, was achieved, equal to the number of copulatory series; Copulatory Activity Duration (CAD) – time interval (s) from the first intromission of a first series to the final ejaculation; the total number of intromissions during the whole copulatory period (ЈIF); the total number of thrusts during the mounts with intromission and ejaculation during all the series (ЈNT). During the experiments, direct observations and filming with a video camera Logitech HD webcam C270, (1280×720 pixels) were conducted.
Statistical analysis
The average value and its errors of copulatory behaviour patterns are calculated and compared using Student's t-test.
Results
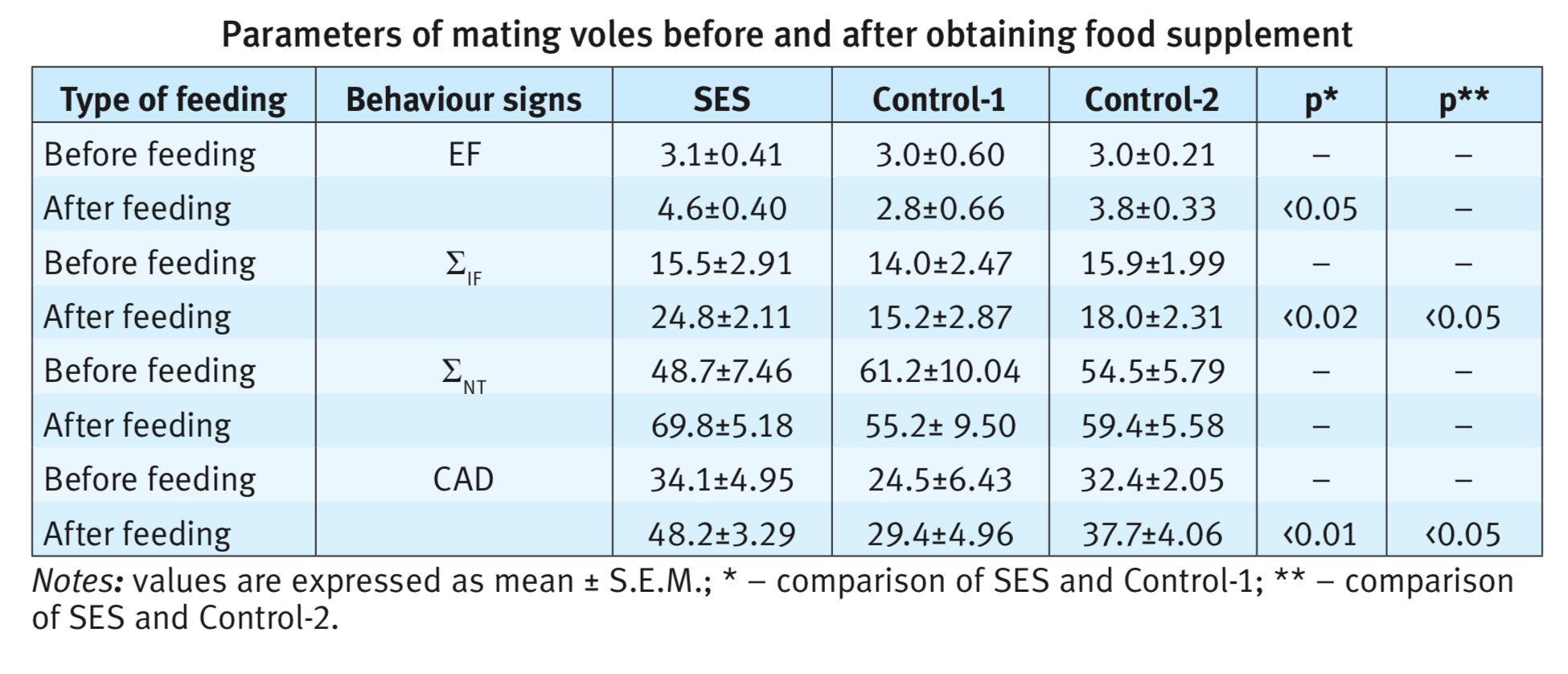
Addition of shiitake mushroom extract to the nutrition of the animals had a pronounced stimulating effect on the parameters of copulatory behaviour of males. Shiitake-fed males showed significant increase the copulation’ indices: EF (p<0,05), ЈIF (p<0,02), ЈNT (p<0,001) и CAD (p=0,001). In SES fed voles, the 30-min criteria for satiety was achieved an average after 14 min, while in Control-1 it is only after 4.9 min and in Control-2 after 5.2 minutes later than in the first experiment before feeding. In SES fed voles such indices as ЈIF, ЈNT and CAD increase (table) in comparison with animals of Control-1 and Control-2 groups.
Differences of copulatory behaviour before and after feeding in Control-1 group are not noted. Only one change in the number of ejaculations (p <0.05) was shown in Control-2 group. The latency of the first intromission in the second copulation test decreases in all three groups (SES – in 1.7, Control-1 – in 1.4 and Control-2 – in 2.2 times). A similar trend was noted in experiments with males of voles, in which a physiological decrease in sexual activity was observed. Already after 7 days of obtaining a food supplement, males increased their sexual motivation and started mating. The most noticeable effect observed in with SES-fed animals, the number of ejaculations varied from 2 to 4 (Fig. 1). On the contrary, in the control groups, one male never began to mate, others only had one ejaculation, and some of the series did not end with ejaculation.
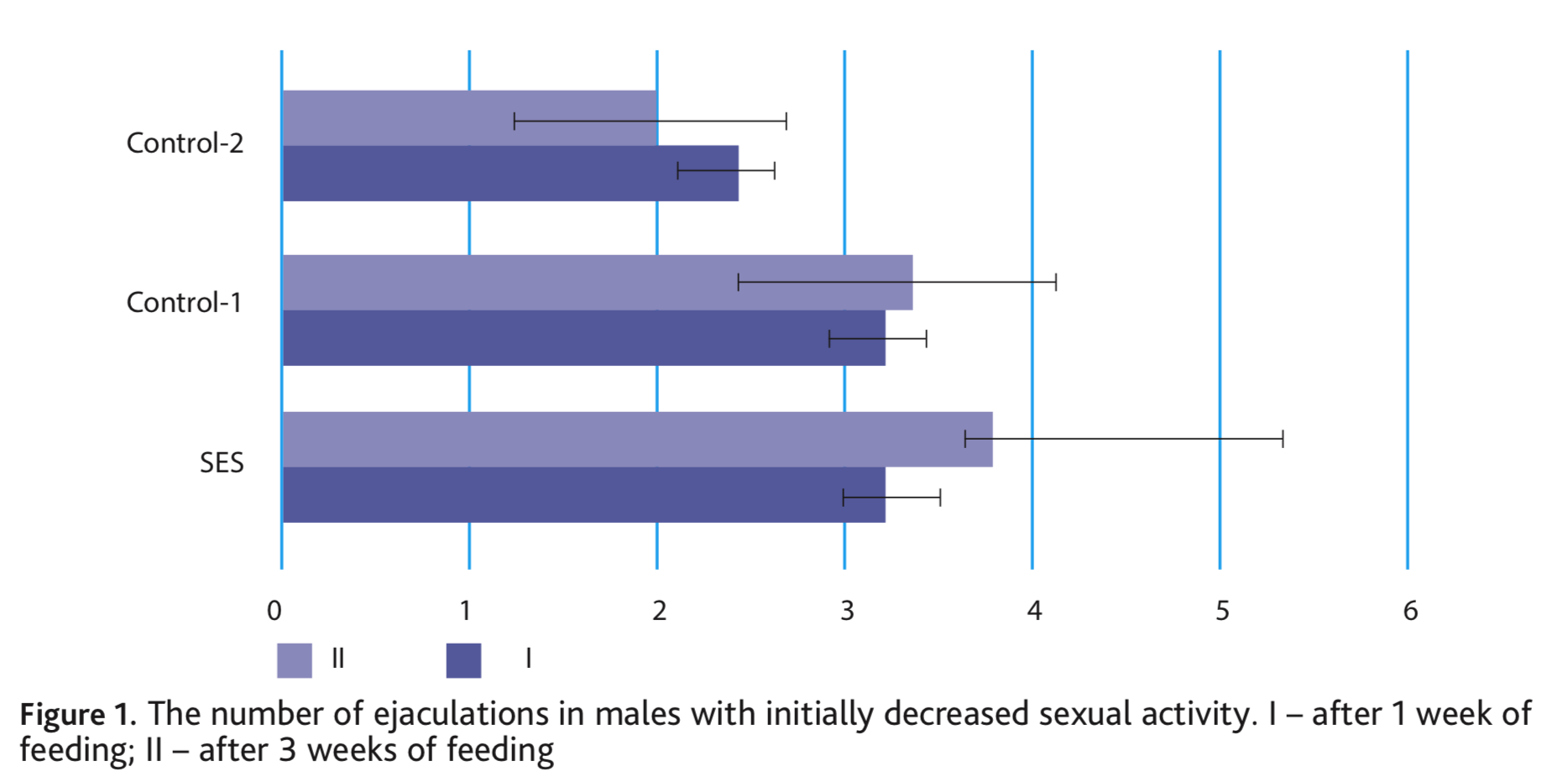
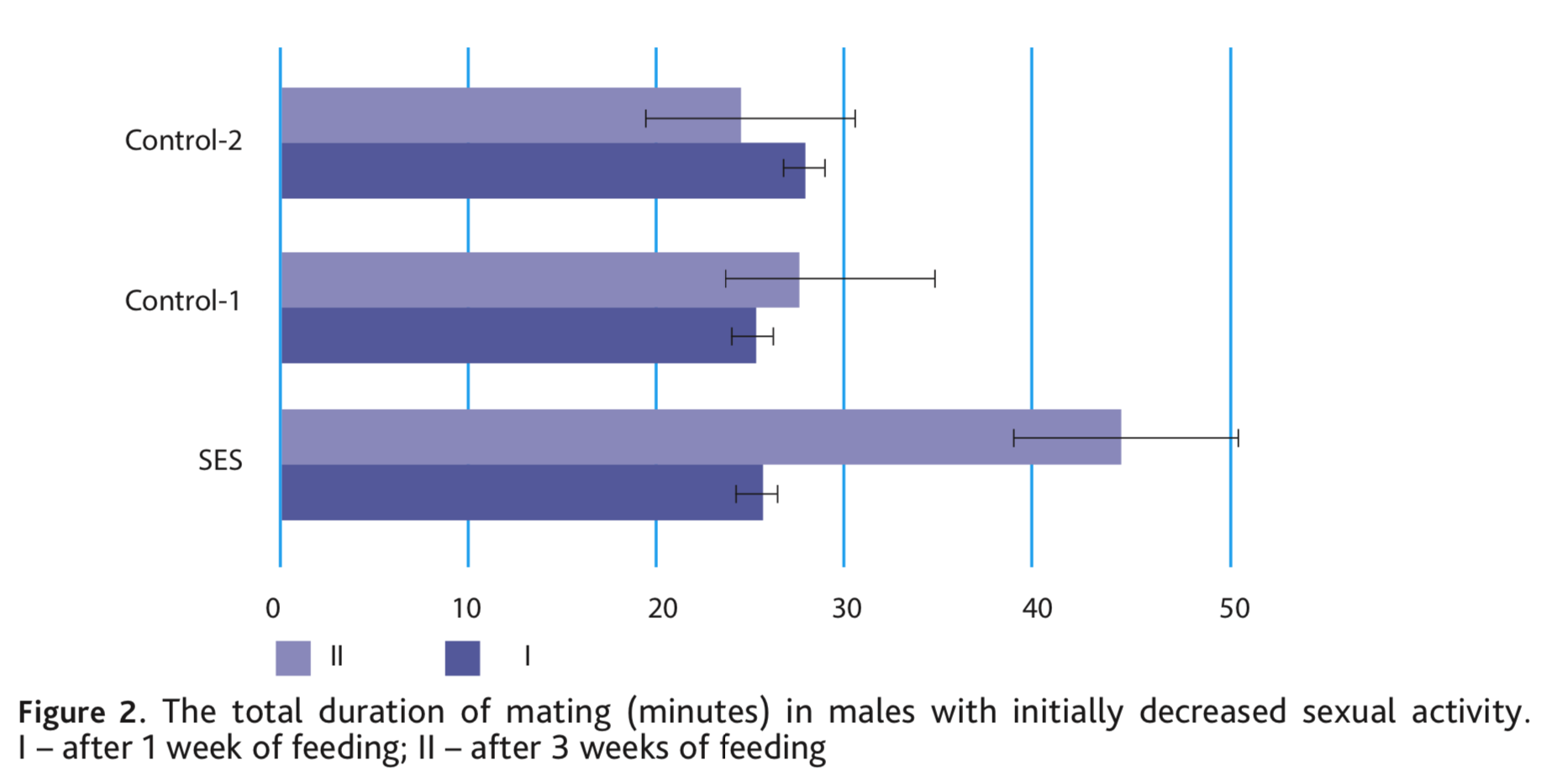
After a three-week diet including nutritional supplements, the most noticeable effect was also observed with SES, on average males increased the number of ejaculations 1.2 times and the differences were significant at p <0.05. In both control groups, there was no increase in ejaculation (Fig. 1). Simultaneously, with the addition of SES, the total duration of mating of the voles increased substantially, by an average of 19.4 min. In the control groups, the duration of mating also did not change (Fig. 2).
Discussion
The stereotype of copulation of voles is species-specific (15, 17), its variability, mainly depends on the individual characteristics of the behaviour of males. M. socialis is characterized by multiple ejaculations, multiple intromissions and thrusts [17, 19]. The number of ejaculations in the studied species varies from 2 to 4 (an average of 3), while the CV is only 22.6%. It is noted that the sexual activity of males differs a little, in some males it is increased, in others it is lowered in relation to the average. Some male may not mates even when kept with a female. Many adaptogens of different origin influence similarly on sexual activity. Caviar of sea urchins, ginseng root tincture and especially pantocrin (deer antler extract) increases the number of ejaculations in rats [20–22].
Oyster mushroom was used as a second control. When the oyster mushroom extract was added to the ration, an increase in the number of ejaculations was shown, but did not affect other mating parameters, including the duration of mating until sexual saturation. Pleurotus ostreatus is recognised as a natural regulator of cholesterol and other lipids, and in addition lowers blood pressure and sugar levels, and has anti-inflammatory properties. Thus, it protects against arterial wall thickening (atherosclerosis), cardiovascular disease and diabetes. The mushroom also strengthens the immune system, has antitumor properties, beneficial effects on the liver, and improves antioxidant status. Pleurotus ostreatus helps prevent age-associated diseases, therefore is considered to have anti-ageing properties, and exhibits antimicrobial and antiviral activities [23–25]. Clinical studies have shown that Pleurotus ostreatus reduces cholesterol and triglyceride levels, without any deleterious effect on the liver and kidney [26]. We can assume that this fungus will also have a little stimulating effect on sexual behaviour.
In voles, there was no significant decrease in mating latency, but an increase in the duration of mating occurred. However, the fruit flies have an inverse relationship. The latency of pairing in males and females of the fruit fly decreased statistically significantly, whereas the duration of pairing did not change significantly [13]. Differences may be due to the peculiarities of the mating strategy of both representatives of vertebrates and invertebrates.
The fertility of two studied species is significantly different. In the vole pairs who received shiitake extract supplement it was 1,4–1,8 times higher than in the control pairs as estimated by the proportion of the females who got involved in mating. The average weight of new-born pups in SES fed pairs increased by 20%. It is shown, placenta and milk mediated stimulation of the development of exterior morphological features and sexual maturity in vole pups, whose parents have received dietary SES [8]. Fruit fly females had no significant effect on fertility [13].
The results obtained in the experiments indicate a high aphrodisiac activity of shiitake mushrooms. A three-week diet supplemented with an aqueous extract of shiitake led not only to the restoration of sexual motivation in males with initially decreased libido, but also to an increase in the number of ejaculations by 1.5 times, and the total duration of mating by 1,8 times. One male doubled the number of ejaculations (6), and other demonstrated 7 ejaculations, i.e. significantly exceeded the average species index. The ration with the addition of wheat bran (Control-1) or the oyster mushroom extract (Control-2), although it restores the attraction of males to the female and mating, but did not change the mating parameters. Moreover, in Control-2 (oyster mushroom) they were lower than in the average norm.
In males with normal sexual capacity, the aphrodisiac effect of the water extract of shiitake was more significant. Males later demonstrated weariness and sexual saturation; ejaculatory activity and genital stimulation were higher (CAD, EF and ЈNT), which is important for more successful fertilization of the female and obtaining a greater number of offspring.
It can be noted that shiitake mushrooms can line up with other adaptogens, the search of which has been actively pursued for many decades [21, 22]. However, the mechanism of action of both plant and animal origin stimulants on the neurochemical processes of libido is not clear as well.
Apparently, the influence of shiitake on the reproductive function of animals is determined by several mechanisms. One mechanism is due to the action of polysaccharides, which are the immune system modulating polysaccharides, especially lentinan (1,3-D glucan) [1, 27]. It has been suggested as substance of the shiitake effect on sexual function [1, 27]. Lentinan causes dilatation of the blood vessels, an important factor of erection. Hot water extraction is one of the main methods used for obtaining material rich in high molecular weight polysaccharides from different natural sources, including shiitake mushroom [28–30]. Shiitake affects the regulation of cholesterol in the body, and has an antisclerotic effect [27, 31]. It is proved that a high level of fats in the blood (hyperaemia) and high blood pressure often causes atherosclerotic changes in the arteries of the penis and, as a result, impotence [32].
According to another hypothesis, the stimulating effect provides from a high level of zinc, the content of which in mushrooms is high [33, 34]. Analysis of the zinc content in the liver and prostate of the vole’ males showed that the prostate contains significantly more zinc (3.7–30.0 mg%) than in the liver (2.8–5.8 mg%) [14]. Zinc is one of the main components of the prostate secretion. An increase in the zinc content in the prostate of the voles, who received shiitake extract during feeding, can explain the results of improving sperm counts such as its concentration and motility of spermatozoa. Shiitake mushrooms and oyster mushrooms contain a relatively high percentage of L-carnitine [35], which is supposed play an important role in stimulating the development of the sexual glands and sexual activity of males [36, 37]. It is providing an energy substrate for the maturation and motility of spermatozoa [38, 39].
Conclusion
For the first time under the conditions of a controlled experiment, the influence of shiitake mushroom extract on the sexual activity of mammals was proved. The results obtained in the animals’ experiments indicate a high aphrodisiac activity of shiitake mushrooms that confirm accumulated data over many centuries from oriental medicine.
Object of the experiment was a natural wild species. It was shown that shiitake can be used in the diet of captive-bred animals for improving their reproductive indices, which could be important, for example, for the conservation and reproduction of rare animal species.
Higher mushrooms (including oyster mushrooms) in general have a positive effect on the mammal’s organism, but not every species of mushroom influences sexual behaviour.
Acknowledgements
We would like to express our great gratitude to researcher Dzintra Zala from the Faculty of Biology, University of Latvia for help in preparing extracts of mushrooms.We are grateful to the reviewers for the extensive comments and fruitful discussions of previous versions of the manuscript.
Список источников
- Chang S.-T., Buswell J.A., Miles P.G. Genetics and Breeding of Edible Mushrooms. Netherlands: Gordon and Breach Science Publishers. 1999; 1–324.
- Hobbs C.R. Medicinal value of Lentinus edodes (Berk.) Sing. (Agaricomycetideae). A literature review. Int. J. Мed. Mushrooms. 2000; 2: 287–302.
- Wasser S.P. Shiitake (Lentinus edodes). Encyclopedia of dietary supplements. Coates P.M, Blackman M.R., Cragg G.M., Levine M., Moss J., White J.D. (editors). New York, Marcel Dekker. 2005; 653–64. DOI: 10.1081/E-EDS-120024880.
- Powell M. Medicinal mushrooms: A clinical guide. East Sussex, Mycology Press. 2010.
- Сozens D.D., Masters R.E., Clark R., Offer J.M. The effect of lentinan on fertility and general reproductive performance of the rat // Tox. Letters. 1981; 9: 55–64.
- Сozens D.D., Hughes E.W., Clark R., Offer J.M. The effect of lentinan on the in uterus embryonic and foetal development of rat and on postnatal development of the F1 offspring. Tox. Letters. 1981; 9: 71–6.
- Сozens D.D., Masters R.E., Clark R. The effect of lentinan on pregnancy of the New Zealand white rabbit. Toxicology Letters. 1981; 9: 65–9.
- Zorenko T., Matjushkova N., Muiznieks I. The effect of mushroom shiitake on ontogenesis and reproduction of the social vole Microtus socialis (Rodentia, Cricetidae). Baltic Journal of Laboratory Animals Science. 2003; 13: 69–77.
- Zorenko T., Motmillere I., Matjuskova N. Does the feeding with shiitake mushrooms increase reproductive capacity of mammals? The 3d International Symposium “Physiological bases for increasing the productivity of mammals introduced in zooculture”. 2005. Petrozavodsk on 27–29 Sept. 2005; 72–4.
- Hwang J.A., Hossain M.E., Yun D.H., Moon S.T., Kim G.M., Yang C.J. 2012. Effect of shiitake [Lentinula edodes (Berk.) Peglerх mushroom on laying performance, egg quality, fatty acid composition and cholesterol concentration of eggs in layer chickens. J. Med. Plants Res. 2012; 1: 146–53.
- Djordjevic B., Škugor S., Jųrgensen S.M., Ųverland M., Mydland L.T., Krasnov A. Modulation of splenic immune responses to bacterial lipopolysaccharide in rainbow trout (Oncorhynchusmykiss) fed lentinan, a beta-glucan from mushroom Lentinula edodes. Fish&Shell fish Immuno. 2009; 26 (2): 201–9.
- Mотмиллере И., Зоренко Т., Матюшкова Н. Улучшение репродуктивных показателей, разводимых в неволе млекопитающих с помощью подкормки их экстрактом гриба шиитаке. Современные проблемы природопользования, охотоведения и звероводства. Международная научно-практическая конференция. Киров. 2007; 303–4.
- Svilpe E., Matjushkova N. Influence of shiitake muschroom Lentinula edodes on reproduction of Drosophila melanogaster. Proceeding of the Latvian Academy of Science. Section B. 2010; 64 (5/6): 223–8. DOI: 10.2478/v10046-010-0008-2.
- Зоренко Т.А. Общественные полевки подрода Sumeriomys (систематика, биология и поведение). 2013; LAP Lambert Academic Publishing: 1–541.
- Dewsbury D.A. Patterns of copulatory behaviour in male mammals. Quarterly Review of Biology. 1972; 47 (1): 1–33.
- Chihara G., Hamuro J., Maeda Y., Arai Y., Fukuoka F. Fractionation and purification of the polysaccharides with marked antitumor activity especially lentinan, from Lentinus edodes (Berk). Cancer Res. 1970; 30: 2776–81.
- Зоренко Т.А., Скиндерская И.А. Половые циклы и поведенческая рецептивность у самок общественных полевок подрода Sumeriomys (Rodentia, Arvicolinae). Зоол. журн. 1996; 75 (10): 1735–47.
- Зоренко Т.А. Морфология гениталий и половое поведение общественных полевок подрода Sumeriomys (Arvicolinae, Microtus). Зоологический журнал. 2000; 79 (8): 990–9.
- Зоренко Т., Мотмиллере И. От чего зависит репродуктивный успех самцов общественной полевки? Поведение и поведенческая экология млекопитающих. Материалы международного совещания, Черноголовка. 2007; 245–8.
- Хотимченко Ю.С., Кропотов А.В., Лисаковская О.В. Природные адаптогены в регуляции половых функций человека и животных. Вестник ДВО РАН. 1996; 5: 15–24.
- Кропотов А.В., Лисаковская О.В., Хотимченко Ю.С. Сезонные особенности влияния адаптогенов на половое поведение экспериментальных животных. Экспериментальная и клиническая фармакология. 2001; 64 (6): 60–2.
- Кропотов А.В., Лисаковская О.В., Юрьева М.И., Плаксен Н.В., Хильченко Н.С., Гончарова Р.К. Влияние адаптогенов или рационов, содержащих икру морских ежей, на половое поведение самцов крыс. Тихоокеанск. мед. журн. 2009; 1: 32–5.
- Gunde-Cimerman N. Medicinal value of the genus Pleurotus (Fr.) P.Karst. (Agaricaless l, Basidiomycetes). International Journal of Medicinal Mushrooms, 1999; 1 (1): 69–80.
- Gunde-Cimerman N., Plemenitaš A. Hypocholesterolemic activity of the genus Pleurotus (Jacq. Fr.) P. Kumm. (Agaricales s. I., Basidiomycetes). International Journal of Medicinal Mushrooms. 2001; 3 (4): 395–7.
- Patel Y., Naraian R., Singh V.K. Medicinal properties of Pleurotus species (oyster mushroom): A review. World Journal of Fungal and Plant Biology. 2012; 3 (1): 1–12.
- Khatun K., Mahtab H., Khanam P.A., Sayeed M.A., Khan K.A. Oyster mushroom reduced blood glucose and cholesterol in diabetic subjects. Mymensingh Medical Journal. 2007; 16 (1): 94–9.
- Flynn V.T., 1991. Is the shiitake mushroom an aphrodisiac and a cause of longevity? In: Maker, M.J. (Ed.), Science and Cultivation of Edible Fungi. Balkema, Rotterdam, pp. 345–61.
- Miles P.G., Chang S-T. Mushroom Biology. Concise Basics and Current Developments. World Scientific, Singapore. 1997: 1–194.
- Zhang M., Cui S. W., Cheung P. C. K., Wang, Q. Antitumor polysaccharides from mushrooms: A review on their isolation process, structural characteristics and antitumor activity. Review. Trends in Food Science & Technology. 2007; 18: 4–19.
- Lee H.H., Lee J.S., Cho J.Y., Kim Y.E., Hong E.K. Structural characteristics of immunostimulating polysaccharides from Lentinus edodes. J. Journal of Microbiology and Biotechnology 2009; 19 (5): 455–61.
- Kabir Y., Yamaguchi M., Kimura S. Effect of shiitake (Lentinula edodes) and maitake (Grifola frondosa) mushrooms on blood pressure and plasma lipids of spontaneously hypertensive rats. Journal of Nutritional Science and Vitaminology. 1987; 33: 341–6.
- Virag R. Is impotence an Arterial Disorder? The Lancet. 1985; 26: 181–4.
- Antoniou L.D., Shalhoub R.J., Sudhavar T. and Smith J.C., Reversal of uremic impotence by zinc. The Lancet. 1977; 2: 895–8.
- Reguła J., Gramza-Michałowska A. Effect of the addition of dried shiitake (Lentinula edodes) to corn crackers on their chemical composition and ability to bind Fe(III) and Zn(II) – a short report. Polish journal of food and nutrition sciences. 2009; 59 (3): 275–8.
- Зоренко Т.А., Мотмиллере И., Дамброва М.У., Матюшкова Н.А. Вероятная роль карнитина в половом поведении самцов общественной полевки. 4-я Всеросс. конф. по поведению животных. М., 2007: 598–9.
- Cavallini G., Cracciolo S., Vitali G., Modenini F., Biagiotti G. Carnitine versus androgen administration in the treatment of sexual dysfunction, depressed mood, and fatigue associated with male aging. Adult Urology. 2004; 63: 641–6.
- Coates P.M, Blackman M.R., Cragg G.M., Agarwal A, Said TM. Carnitines and male infertility. Reproductive BioMedicine Online. 2004; 8: 376–84.
- Sinclair S. Male infertility: nutritional and environmental considerations. Alternative Medicine Review. 2000; 5: 28–38.
- Ahmed S.D., Karira K.A, Jagdesh, Ahsan S. Role of L-carnitine in male infertility. Journal of Pakistan Medical Association. 2011; 61 (8):732–6.




